Intern Ultrasound of the Month: Aortic Dissection
The Case
55-year-old male with history significant for CVA with residual left-sided weakness, hypertension, and prior aortic arch and valve replacement who presented for 5 days of worsening left-sided weakness to the point that he was unable to get up from bed. He denied any other symptoms or recent trauma. On arrival to the ED, he was tachycardic but normotensive and had otherwise normal vitals. Exam was notable for weakness in his left upper > lower extremity, along with diminished sensation.
Labs, EKG, CT head and CTA were ordered. POCUS was performed to evaluate for aortic pathology.
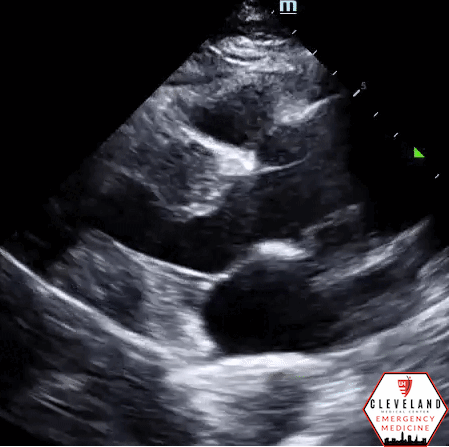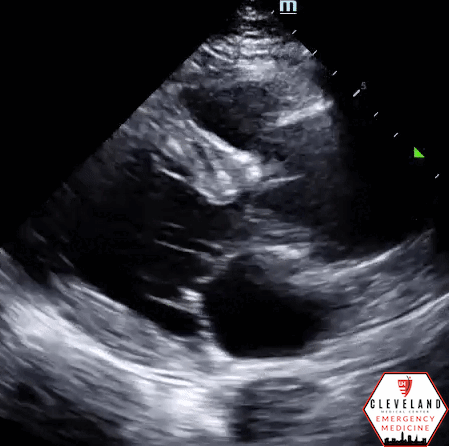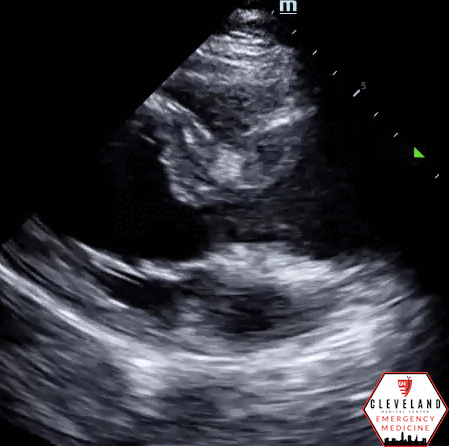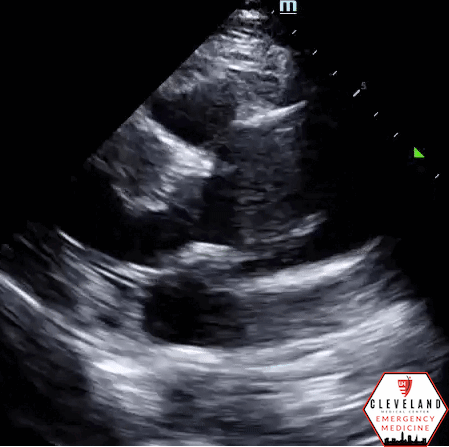
Abdominal aorta transverse view — hyperechoic intimal flap; no apparent aneurysm
Abdominal aorta long axis — hyperechoic intimal flap; no apparent aneurysm
Parasternal long axis view - no aortic root dilation, pericardial effusion, or visible dissection flap
Suprasternal view - descending part of the aortic arch. There’s a hyperechoic dissection flat at the proximal aspect of left subclavian artery.
POCUS Findings
Intimal dissection flap within the abdominal aorta. No evidence of aortic root dilation, pericardial effusion, or obvious intimal flat on cardiac views (PSLA shown here). The suprasternal notch view is somewhat limited (you primarily see the descending part of the arch) but there is a thin dissection flap at the branch point of the subclavian artery. Findings are suggestive of aortic dissection.
Case continued
CT showed a type A dissection extending from from the origin of the innominate artery to the bifurcation of the abdominal aorta. He was evaluated by cardiac and vascular surgery and deemed a poor candidate for surgical revision; therefore, his dissection was managed medically. He had a fairly uncomplicated hospital course and was ultimately discharged home.
Aortic Dissection
What is it?
Tear in the layers of the aorta separating the intimal and medial layers with blood entering between the two, creating a true lumen and false lumen
Classification:
Type A: ascending aorta
Type B: descending aorta
Epidemiology
Aortic dissection has a reported incidence of 5-30 per 1,000,000 people per year [1], most commonly occurs in the 7th decade of life with a 1.5:1 male predominance [2]. By comparison, acute MI’s occur at a rate of roughly 4400 per 1,000,000 people per year [1].
While relatively rare, dissection represents a “can’t-miss-diagnosis” with an overall mortality rate of 1-2% per hour immediately after symptom onset if untreated. Type A dissections have an in-hospital mortality rate exceeding 50% if managed medically [3].
Approximately 77% of patients with aortic dissection have a history of hypertension, which is the most common risk factor [3]. Other major risk factors include atherosclerosis (31%), prior cardiac surgery (18%), and known aortic aneurysm (16%) [1]. Cocaine use and strenuous activity that may result in abrupt increases in blood pressure (ie weight lifting) have also been implicated [4,5].
Connective tissue diseases, such as Marfan’s, represent a minority of dissection cases overall but notably account for as many as 50% of cases presenting before the age of 40 [6].
Clinical Presentation
Abrupt onset chest/back/abdominal pain that is severe or “worst ever” should raise your suspicion for dissection. In one study of 464 patients with confirmed type A dissection, 90% of patients reported severe or worst ever pain, with abrupt onset reported in 85%. Other classic descriptors such as sharp, tearing/ripping, and migrating were less reliable [1].
Chest pain is more commonly reported among patients with type A dissections (85% vs 67% for type B), while back pain is more common in type B dissections (70% vs 43% for type A) [7].
Classic exam findings such as pulse deficits or focal neurologic deficit (in conjunction with pain) can help rule in dissection (+LR ~6) [8] but their absence cannot rule out diagnosis as they are present in only 11% and 20%, respectively [9].
While patients with type B dissection often present hypertensive, the majority of patients with type A dissection present normotensive or hypotensive [3].
Diagnostics
CXR is classically known to show a widened mediastinum, however this finding is present in roughly half of type A dissections and less frequently in type B [7].
CT angiography (CTA) boasts a sensitivity and specificity nearing 100% and is considered the imaging modality of choice due to accuracy, availability, and speed of exam [10]. Despite the widespread availability of CT, the median door to diagnosis time is reportedly 4.3 hours. Delays have been reported more frequently in women or patients with atypical presentations (ie lack of sudden/severe pain). Additionally, the presence of signs/symptoms suggestive of a more common alternative diagnosis, such as CHF exacerbation severe secondary to aortic regurgitation or STEMI on an EKG resulting from dissection involving the coronary osteum, can be misleading and also lead to delays in diagnosis [3].
MRI and trans-esophageal echo (TEE) are highly accurate but time-consuming and not always readily available. Trans-thoracic echo (TTE) is specific but not sensitive [10].
POCUS for Aortic Dissection
Why POCUS?
POCUS allows for significantly faster time to diagnosis as well as reduced rates of misdiagnosis in the ED [11]. A prospective trial by Wang et al. found POCUS to have a door-to-diagnosis time of 10 minutes (compared to 79 minutes in the non-POCUS group) with a specificity of 100%, similar to CTA [12]. Not bad for a diagnosis where minutes truly matter!
For ascending aortic dissections, the presence of direct findings (i.e. dissection flap) are highly specific while indirect signs (e.g. aortic dilation, pericardial effusion, aortic regurgitation) should raise your suspicion for dissection and prompt you to consider CTA in the right context (if you weren’t already). Of the indirect signs, aortic dilation was most sensitive while pericardial effusion and aortic regurgitation were more specific. Note that the absence of POCUS findings does not rule out dissection [13].
Technique [11, 13-15]
-Cardiac assessment-
Phased-array probe
Obtain a parasternal long axis view — probe marker pointing toward the patient’s right shoulder (in cardiac preset mode)
Evaluate for the following:
Direct findings of dissection
Intimal flap — linear hyperechoic structure moving with each pulsation, separating a true lumen and false lumen
Don’t forget to look at the descending aorta posterior to the heart in the PSLA view (you never know when you might find a dissection flap there)
*Indirect signs*
Aortic root dilation — diameter > 4 cm is considered abnormal (but depending on the specific point of measurement, the precise cutoff of normal will vary)
Measure leading edge to leading edge at end-diastole
A widened aortic root will be disproportionately larger than the RVOT and LA. Normally, the RVOT, aortic root and LA should be in roughly a 1:1:1 ratio.
**Be sure to get a good visualization of the aortic outflow tract/root, otherwise this may result in a false positive or negative interpretation
Pericardial effusion
Aortic regurgitation — apply color doppler over the aortic valve and look for regurgitant jet
Type A Dissection - dilated root with dissection flap
>4 cm is considered abnormal
Normally, you should see a 1:1:1 ratio
-Suprasternal notch assessment-
Position patient with neck extended (off of bed or with pillow under shoulders) to optimize view of aortic arch
Place phased array probe within the suprasternal notch with probe marker pointing toward the patient’s left scapula. Angle the probe obliquely (aimed slightly caudally to obtain a long axis view of the arch)
Evaluate for dissection flap and dilation, as well as abnormal flow using color doppler. Normally, you should see red in the ascending (screen left) and blue in the descending (screen right) aorta as this indicates flow toward and away from the probe, respectively. A dissection may alter this.
Suprasternal notch view. Image from Ma & Mateer [14]
-Abdominal assessment-
Curvilinear or phased array probe, abdominal preset
Place probe just caudal to patient’s xyphoid with probe marker pointing toward the patient’s right.
Adjust depth to visualize the vertebral body (look for the distinct vertebral shadow) posteriorly with aorta just anterior to this. Don’t confuse more superficial anechoic structures for the aorta.
Slowly slide the probe caudally, attempting to visualize the aorta throughout its course (proximal, mid, distal aorta), until you visualize the bifurcation into the iliac arteries.
Once you’ve completed the transverse assessment, rotate the probe 90° so the probe marker is now pointed toward the patient’s head to achieve a long axis view of the aorta.
Pitfall: views may be limited by bowel gas. Attempt graded compression to help with this.
Evaluate for the following:
Direct
Intimal flap — hyperechoic linear flap moving with each pulsation, dividing the lumen into a true and false lumen
Intramural hematoma — echogenic structure within the lumen
Indirect
Aneurysm — diameter > 3 cm aorta or > 1.5 cm iliac artery (outer wall to outer wall)
Irregular flow with color doppler — may see bidirectional flow or antegrade flow in the true lumen with poor/absent flow in the false lumen
Take Home Points
Aortic dissection is a deadly pathology with variable presentation. Risk factors include hypertension, prior cardiac surgery, and connective tissue diseases such as Marfan’s.
Sudden, severe chest/back pain should raise your concern for dissection. While unequal pulses or neuro deficits may be suggestive of dissection, they are poorly sensitive and should not be relied upon to rule out disease.
CTA is the gold standard, but POCUS can quickly evaluate for both direct and indirect findings of dissection (among other pathologies). If abnormal findings are present, POCUS has demonstrated good diagnostic accuracy and can expedite time to diagnosis while reducing misdiagnosis in the ED. However, POCUS has its limitations and should not be used as a stand alone or rule out test for dissection.
AUTHORED BY: DR. RICH DOWD (R1)
FACULTY CO-AUTHOR/EDITOR: LAUREN MCCAFFERTY, MD
References
Hagan P, Nienaber C, Isselbacher E, Bruckman D, Karavite DJ, Russman PL, et al. The international registry of acute aortic dissection (IRAD): New insights into an old disease. JAMA. 2000; 283(7): 897-903.
Meszaros I, Morocz J, Szlavi J, Schmidt J, Tornoci L, Nagy L, Szep L. Epidemiology and clinicopathology of aortic dissection. CHEST. 2000; 117(5):1271-8.
Evangelista A, Isselbacher E, Bossone E, Gleason TG, Di Eusanio M, Sechtem U, et al. Insights from the International Registry of Acute Aortic Dissection: A 20-year experience of collaborative clinical research. Circulation. 2018; 137(17):1846-1860.
Singh A, Khaja A, Alpert MA. Cocaine and aortic dissection. Vasc Med. 2010;15(2):127-133.
Hatzaras I, Tranquilli M, Coady M, Barrett PM, Bible J, Elefteriades JA. Weight lifting and aortic dissection: more evidence for a connection. Cardiology. 2007;107(2):103-106.
Januzzi J, Isselbacher E, Fattori R, Cooper JV, Smith DE, Fang J, et al. Characterizing the young patient with aortic dissection: results from the international registry of aortic dissection (IRAD). J Am Coll Cardiol. 2004; 43 (4) 665-669.
Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015; 28;66(4):350-8.
Klompas M. Does this patient have an acute thoracic aortic dissection?. JAMA. 2002;287(17):2262-2272.
Rogers AM, Hermann LK, Booher AM, Nienaber CA, Williams DM, Kazerooni EA, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123(20):2213-2218.
Vardhanabhuti V, Nicol E, Morgan-Hughes G, Roobottom CA, Roditi G, Hamilton MCK, et al. Recommendations for accurate CT diagnosis of suspected acute aortic syndrome (AAS)--on behalf of the British Society of Cardiovascular Imaging (BSCI)/British Society of Cardiovascular CT (BSCCT). Br J Radiol. 2016; 89(1061):20150705.
Pare JR, Liu R, Moore CL, Sherban T, Kelleher MS, Thomas S, et al. Emergency physician focused cardiac ultrasound improves diagnosis of ascending aortic dissection. Am J Emerg Med. 2016;34(3):486-492.
Wang Y, Yu H, Cao Y, Wan Z. Early Screening for Aortic Dissection With Point-of-Care Ultrasound by Emergency Physicians: A Prospective Pilot Study. J Ultrasound Med. 2020;39(7):1309-1315.
Nazerian P, Mueller C, Vanni S, de Matos Soeiro A, Leidel BA, Cerini G, et al. Integration of transthoracic focused cardiac ultrasound in the diagnostic algorithm for suspected acute aortic syndromes. Eur Heart J. 2019;40(24):1952-1960.
Reardon RF, Laudenbach A, Joing SA. Cardiac. In OJ Ma, JR Mateer, RF Reardon, SA Joing (eds), Ma and Mateer’s Emergency Ultrasound (3rd ed). 2008. New York, NY: McGraw-Hill Education. pp 93-167.
https://www.coreultrasound.com/ad/