Intern Ultrasound of the Month: A Painful, Swollen Knee
The Case
85-year-old male with past medical history including hypertension and atrial fibrillation (for which he was on Eliquis) presented to the ED for atraumatic right knee pain and swelling. He had been having increasing difficulty walking on the knee for the last few days; however, on the day of presentation, he noticed that any small movements caused severe pain. He denied any recent falls or trauma or previous injury to knee. He had never had these symptoms before and had no history of gout or IV drug use. He denied fevers, chills, nausea, vomiting, or recent dental work.
On arrival to the ED, his vital signs were the following: Temp 98.4, HR 91, RR 18, SpO2 94% on room air, and BP 153/100. Physical exam was notable for a well-appearing male in mild distress secondary to pain. His right knee was swollen and tender to the touch. He had significant pain on passive range of motion that knee and was unable to fully bend the knee due to pain and swelling. No obvious skin changes, rash, or overlying erythema. His distal pulses were intact bilaterally.
In addition to obtaining labs (including inflammatory markers) and x-rays to evaluate for possible etiology, point-of-care ultrasound (POCUS) of the knee was performed to assess for joint effusion prior to performing a diagnostic arthrocentesis to evaluate for septic arthritis.
POCUS Findings:
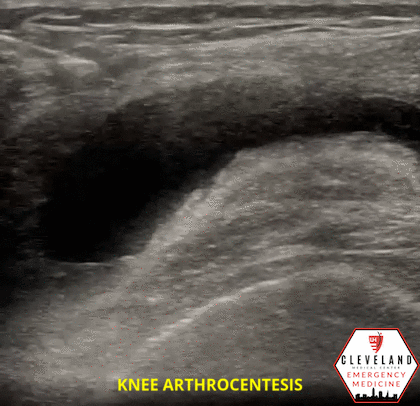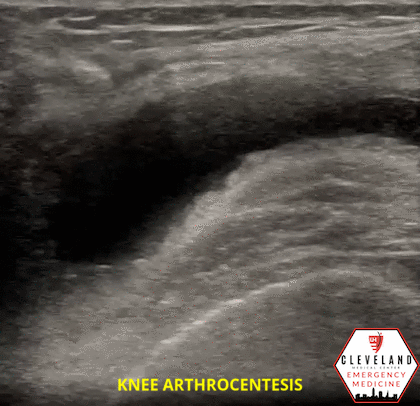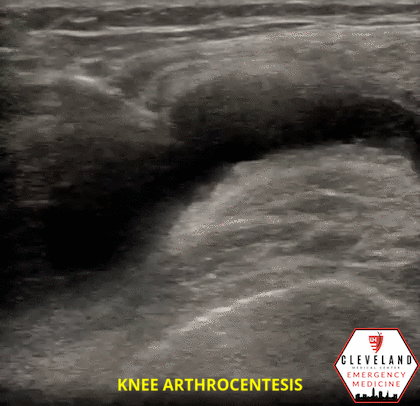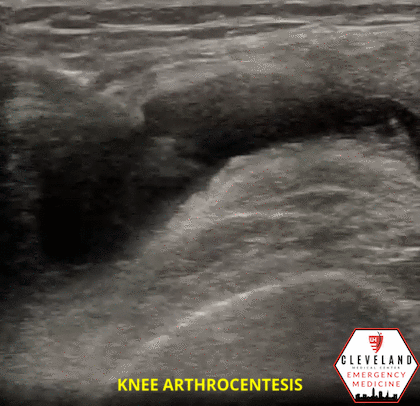
A moderate volume of hypoechoic synovial fluid with some echogenicity is seen within the right knee joint with the largest area of fluid in the superolateral region. There was no major vasculature present in the area of planned needle entry for arthrocentesis.
Case continued:
X-ray of the knee showed no acute fracture or malalignment, but there was a moderate nonspecific knee effusion noted. CBC and CMP were unremarkable. He did have an elevated CRP of 2.1 and elevated ESR of 34.
A diagnostic ultrasound-guided arthrocentesis (as described below) was performed without complication. Approximately 35 cc’s of viscous sanguinous fluid was aspirated from the knee joint. Fluid studies including cell count, culture, crystal analysis and gram stain were obtained. Fluid results revealed: WBC 5100, red color, cloudy clarity, RBC 3,375,000, Neutrophils 75, Lymphocyte 10,200. No crystals were seen on microscopy, and no organisms were seen on gram stain. There was no growth aerobically or anaerobically on fluid culture.
Based on these results, the patient was diagnosed with hemarthrosis, thought to be secondary to his anticoagulant use. The joint was wrapped with an ACE bandage and elevated. The patient was comfortable with discharge and was instructed to follow up with his primary care physician.
POCUS in the Evaluation and Management of Joint Effusions
Overview
Historically, joint arthrocentesis has been performed based on physical exam and landmark identification. With the ever-increasing popularity of ultrasound, many providers have started utilizing ultrasound for effusion identification, optimal location for aspiration, and assistance of needle-guidance, which is especially useful for smaller volume effusions. The traditional landmark-based identification method has limitations due to variations in anatomy and effusions. Success rates range from 61-78% depending on the joint involved [1]. Ultrasound offers several advantages, diagnostically and procedurally, by allowing for direct visualization of the effusion. Ultrasound can identify joint effusions as small as 4cc, which are often difficult to palpate on physical exam [2]. It is also useful in differentiating effusions from soft tissue abnormalities, which can reduce unnecessary joint aspirations [3]. Ultrasound guidance for arthrocentesis leads to greater accuracy and lower pain scores both intra- and post-procedurally compared to the traditional landmark-based approach [4]. In novice providers performing arthrocentesis, the use of ultrasound increased first-attempt success over landmark-based techniques, particularly for smaller volume effusions [5]. It has also been shown to result in more fluid aspiration and greater novice provider confidence while not taking any longer than standard palpation alone [6].
Figure 1. Sonographic knee anatomy with anechoic (black) fluid identified underneath fat pad
Confirming Joint Effusion
When utilizing ultrasound to assist in the evaluation of a swollen knee joint, use the high-frequency linear probe to obtain longitudinal and transverse views of the knee. For the longitudinal (i.e. sagittal) view, place the probe just above the patella with the probe marker aimed cephalad. The suprapatellar window should include the superior portion of the patella, the quadriceps femoris tendon (long axis), fat pad, and femur. An effusion will be located between the quadriceps tendon and femur, immediately below the fat pad. Depending on the effusion, the fluid will typically appear as hypoechoic, however, it can vary, depending on the type of effusion. To obtain a short axis, or transverse, view, simply rotate the probe 90 degrees [1, 7]. Additional views can be obtained by scanning along the medial and lateral borders of the patella.
Determining Optimal Site for Arthrocentesis & Minimizing Complications
The optimal site for arthrocentesis is generally where the fluid collection is largest and best visualized; this is often the suprapatellar space. Once the best site for needle aspiration is identified, apply color Doppler to the area to ensure there are no vessels within the planned needle trajectory; this is especially important in patients on anticoagulation or with bleeding disorders. To reduce risk of infection, it important to perform this under fully sterile conditions [1].
Static vs Dynamic Ultrasound-Guidance
Once the above steps are taken, providers can choose whether they want to proceed with a static versus dynamic ultrasound-guided technique. With a static technique, ultrasound is used to identify the optimal site for needle entry but the remainder of the procedure is performed without it. Conversely, a dynamic technique involves using ultrasound in realtime to directly guide the needle into the fluid [8]. By allowing the provider to visualize and redirect the needle trajectory to the appropriate joint space, this technique is especially helpful when there is a smaller effusion, a smaller or difficult-to-reach joint space, or the nearby presence of a neurovascular bundle or other important structure(s) to avoid [9]. This technique is demonstrated below.
In-plane vs Out-of-plane Approach
Both in-plane and out-of-plane techniques are described in the literature, though the former is often described as the more preferred technique [1,7]. The in-plane technique refers to inserting the needle at the leading edge of the probe and advancing directly under and parallel to the transducer, thus allowing for visualization of the full length of the needle along its trajectory. The out-of-plane technique refers to inserting the needle perpendicular to and at the center of the transducer, allowing visualization of only a small part of the needle at any given time; ideally, you only visualize the needle tip to help ensure you do not advance the tip beyond the probe [8].
Figure 2. In-plane vs out-of-plane [Macias]. From The International Journal of Shoulder Surgery
Figure 3: In plane technique of knee arthrocentesis supralateral approach [Johnson]
Utility of Ultrasound in Arthrocentesis of Additional Joints
We chose to focus on knee arthrocentesis based on our patient’s presentation. However, ultrasound also provides diagnostic and procedural utility for joint effusions involving the ankle, hip, shoulder, elbow, and wrist, as well as additional smaller joints. Ultrasound is especially advantageous for smaller or less accessible joints given their more limited access and allows for greater accuracy than a palpation-guided approach with increased initial intra-articular success [10]. Similar to the knee, the high frequency linear probe remains the most commonly recommended probe.
The Procedure
Materials
Sterile US probe cover and gel
18g needle attached to 10 cc (or larger) syringe
Chlorhexidine
Sterile drape and sterile gloves
Sterile syringe and needle with lidocaine
Figure 4. Arthrocentesis video demonstrating in-plane approach with the transducer in transverse orientation
Dynamic Ultrasound-Guidance
Identify the optimal location by evaluating with ultrasound as described above
Place patient (and yourself) in the optimal position; have patient’s knee slightly flexed to improve fluid visualization
***Ensure US machine is positioned in direct line of site if using dynamic guidance
Clean area and apply sterile field, place US transducer in sterile probe cover and lay on sterile field
Anesthetize the local subcutaneous tissues using lidocaine
While multiple techniques can be used, we recommend an in-plane approach to allow for direct visualization of the needle. As previously discussed, the needle should enter the skin at the leading edge of the linear transducer
Visualize your needle and advance toward the effusion, making small redirections as needed. If you lose site of the needle, realign the probe with the needle to ensure good visualization of the needle shaft before advancing any further
Apply gentle negative pressure with the syringe until aspiration of synovial fluid is achieved
Pearls and Pitfalls
Always perform under sterile conditions; this includes using a sterile probe cover when using dynamic ultrasound-guidance to reduce risk of contamination/infection
Position the patient for increased success, i.e. ensure the knee is slightly bent to open the joint space
Color Doppler can be used to help identify vascular structures
Needle-finding skills are user dependent and moving the transducer during procedures can lead to difficult needle visualization
Evaluation of synovial fluid
The evaluation of synovial fluid is the gold standard for excluding septic arthritis in patients with concerning presentation. Arthrocentesis also allows for further determination of the cause of joint effusions. Labs including CBC, CRP, and ESR are often obtained prior to arthrocentesis; however these tests do not sufficiently lower the post-test probability enough to avoid obtaining synovial fluid for analysis [11].
Figure 4 presents a step-wise approach for synovial fluid analysis. Begin by analyzing the gross appearance, including color, clarity and viscosity. Determine if the effusion is hemorrhagic, then evaluate the WBC count to determine if it is inflammatory versus non-inflammatory. Next, evaluate for crystals. Follow-up with gram stain and culture [12].
Figure 5. Algorithm for synovial fluid analysis. Created by Dr. Kelsie Rhyne, adapted from UpToDate [12]
Figure 6. Synovial fluid analysis [14]
Typical evaluation includes synovial gram stain, culture, protein, LDH, glucose and lactate [11-13]. There are some variations for cutoff ranges of labs in synovial fluid findings. Synovial fluid WBC count >50 x 10^9/L is concerning for septic arthritis, though this can also be seen in inflammatory arthropathies, including gout, RA, and pseudogout, or in immunocompromised states. Typical guidelines suggest a WBC <200 is normal, 200-2,000 is non-inflammatory, 2,000-50,000 is inflammatory, and > 50,000 is infectious, although upper limit cutoffs in septic arthritis are not clearly established [12-13]. It’s also worth mentioning that synovial WBC counts can be lower than expected in cases of septic arthritis, and any true concern should be treated as assumed positive while further workup is pending. The fluid must also be analyzed for RBC count and crystals. High RBC counts are suggestive of hemarthrosis if remaining labs are otherwise unremarkable. Monosodium urate (needle-shaped) crystals versus calcium pyrophosphate (rhomboid-shaped) crystals will be seen in cases of gout and pseudo-gout, respectively [13]. Cultures of the synovial fluid are considered the most important test and should be obtained for all patients who undergo arthrocentesis. Cultures allow for identification of bacterial organisms, particularly for non-gonococcal septic arthritis [11, 13]. Figure 5 [14] presents a table summarizing findings of different inflammatory versus non-inflammatory arthropathies.
Conclusion
Overall, there are no validated clinical decision tools to help with diagnosing septic arthritis. It’s important to combine the history, physical exam, imaging and lab analysis in order to help decide next steps in care. When considering a septic joint as part of your differential, ALWAYS perform an arthrocentesis.
Take Home Points
POCUS is a valuable bedside tool for quickly evaluating and managing patients with a swollen joints
Diagnostically, ultrasound can quickly confirm the presence of a joint effusion and differentiate from other soft tissue pathology
Synovial fluid analysis is key in diagnosing septic arthritis, so getting comfortable with arthrocentesis and the different techniques is key
By allowing for direct visualization of the fluid and the needle, ultrasound guidance for arthrocentesis leads to greater accuracy, first-pass success, and provider confidence and reduces complications and pain scores, while not significantly affecting duration of the procedure
POST BY: DR. KELSIE RHYNE, PGY1
FACULTY EDITING BY: DR. LAUREN MCCAFFERTY
References
Puebla DL, Farrow RA. Ultrasound Guided Arthrocentesis. StatPearls. Treasure Island (FL): StatPearls Publishing. 7 Aug 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK573084/
Hong BY, Lee JI, Kim HW, Cho YR, Lim SH, Ko YJ. Detectable threshold of knee effusion by ultrasonography in osteoarthritis patients. Am J Phys Med Rehabil. 2011; 90(2):112-8.
Adhikari S, Blaivas M. Utility of bedside sonography to distinguish soft tissue abnormalities from joint effusions in the emergency department. J Ultrasound Med. 2010 Apr; 29(4):519-26.
Wu T, Dong Y, Song Hx, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: A systematic review. Semin Arthritis Rheum. 2016; 45(5):627-32. [PubMed]
Delesky EM, Gaughan J, Roberts B, Sodhi S. Comparison of knee arthrocentesis first-attempt success between Ultrasound-Guided, Ultrasound-Localised and Landmark-Guided techniques in the novice: A crossover study with random order of events. Australas J Ultrasound Med. 2022; 25(2):74-79.
Wiler JL, Costantino TG, Filippone L, Satz W. Comparison of ultrasound-guided and standard landmark techniques for knee arthrocentesis. J Emerg Med. 2010 Jul7; 39(1):76-82.
Johnson B, Lovallo E, Mantuani D, et al. How to perform ultrasound-guided knee arthrocentesis. ACEPNow. 2015; 34(8):16-7.
Macias M. Ultrasound Leadership Academy: Introduction to procedural ultrasound. EM Curious. 20 December 2014. Retrieved January 20, 2023, from http://www.emcurious.com/blog-1/2014/12/7/ultrasound-leadership-academy-introduction-to-procedural-ultrasound
Balint PV, Kane D, Hunter J, et al. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: A pilot study. J Rheumatol. 2002;29:2209-2213.
Raza K, Lee CY, Pilling D, Heaton S, Situnayake RD, Carruthers DM, et al. Ultrasound guidance allows accurate needle placement and aspiration from small joints in patients with early inflammatory arthritis. Rheumatology (Oxford). 2003; 42(8):976-9.
Long B, Koyfman A, Gottlieb M. Evaluation and Management of Septic Arthritis and its Mimics in the Emergency Department. West J Emerg Med. 2019; 20(2):331-341.
Sholter, DE, Russell AS. (2022). Synovial Fluid Analysis. UptoDate. Available from: https://www.uptodate.com/contents/synovial-fluid-analysis?search=synovial%20fluid%20analysis&source=search_result&selectedTitle=1~61&usage_type=default&display_rank=1#H1
Springer BL, Pennington BM. Joint Arthrocentesis in the Emergency Department. Relias Media. 1 Oct 2017. Retrieved from <https://www.reliasmedia.com/articles/141511-joint-arthrocentesis-in-the-emergency-department>.
Nickson C. Synovial fluid analysis. Life in the Fast Lane • LITFL. 2020 November 3. Retrieved January 20, 2023, from https://litfl.com/synovial-fluid-analysis/