Intern Ultrasound of the Month: Cardiac Tamponade
The Case
A 60-year-old female with a past medical history of metastatic breast cancer currently receiving chemotherapy presented to the emergency department for dyspnea on exertion and generalized fatigue. She was only able to take a few steps prior to becoming significantly short of breath but denied any associated chest pain. She reported some occasional palpitations and chronic generalized pain, unchanged from baseline.
Vital signs were notable for tachycardia, tachypnea, and hypoxia, but she had a stable blood pressure and was afebrile. She was in mild respiratory distress and had diminished breath sounds throughout. Cardiac exam was remarkable for tachycardia with mild jugular venous distention present. There was 2+ pitting edema in the bilateral lower extremities and the patient was mentating appropriately with an otherwise non-focal neurologic exam. She was placed on 2 liters of oxygen via nasal cannula and her hypoxia was corrected.
The initial concern was for pulmonary embolism (PE) in the setting of active cancer and chemotherapy and a CT angiogram of the chest was obtained, but a pericardial effusion was also the differential. An EKG was obtained and showed tachycardia without evidence of ischemia but did show subtle electrical alternans.
A cardiac point-of-care ultrasound (POCUS) was performed with the following images obtained:
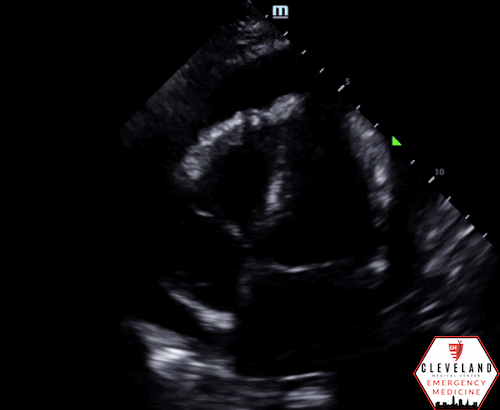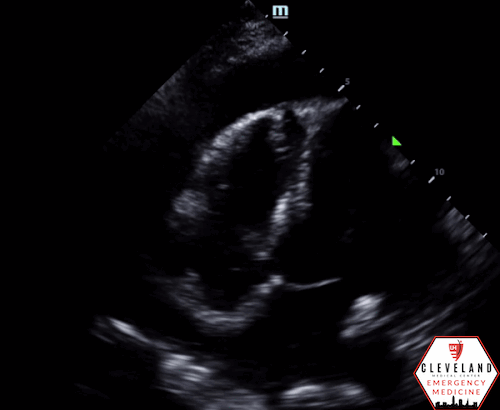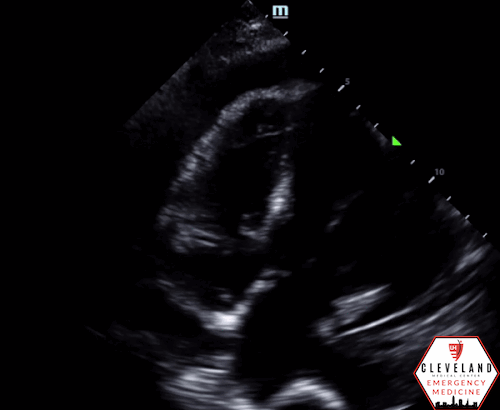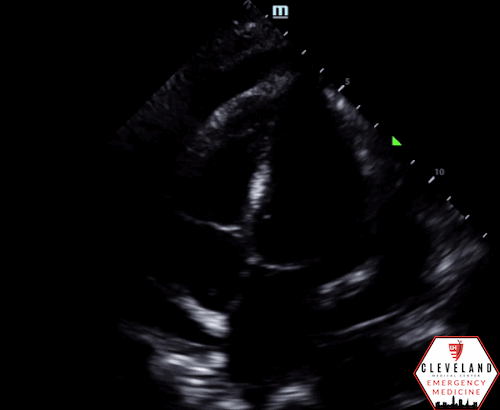
POCUS Findings: There is a large circumferential pericardial effusion with right ventricular systolic collapse and left atrial systolic collapse, indicating tamponade physiology. The left ventricular function appears preserved and there is no right-sided chamber enlargement.
Cardiac Tamponade
Epidemiology
Cardiac tamponade is an uncommon diagnosis, occurring in five out of every 10,000 medical admissions (1). The most common causes of cardiac tamponade include complications from percutaneous interventions (33%), postpericardiotomy syndrome (15%), neoplasm (15%), inflammatory processes such as pericarditis (12.5%), uremia (2%), and acute myocardial infarction (1.5%) (2).
Pathophysiology
Cardiac tamponade occurs when fluid accumulates within the pericardial sac, leading to an increase in pressure that exceeds the pressure required for the right heart to fill during diastole. This results in decreased compliance of the right ventricle, impairing its ability to adequately receive blood during diastole and reducing preload to the left heart. Consequently, this leads to a decrease in cardiac output, which worsens as the pressure in the pericardial sac rises. If left untreated, cardiac output can decrease to a level where myocardial perfusion is inadequate, potentially resulting in cardiac arrest (3,4).
The volume of pericardial fluid required to overcome the diastolic filling pressure depends on the chronicity of the effusion. In a healthy state, the pericardial sac generally contains 15-50 mL of plasma ultrafiltrate (3). If fluid accumulates suddenly - such as from traumatic aortic or cardiac rupture, aortic dissection, or coronary vessel rupture - the diastolic filling pressure can be easily overcome with as little as an additional 50 mL of fluid. Conversely, if fluid builds up gradually, cardiac tamponade may not occur until up to 2 liters are present. Common causes of subacute cardiac tamponade include viral syndromes, uremia, or neoplasms (4,5). Recognizing the factors influencing the onset of cardiac tamponade is crucial for timely intervention and improved patient outcomes.
Clinical Presentation
The typical clinical presentation of cardiac tamponade is highly dependent on the chronicity of the pericardial effusion. Patients with chronic effusions, i.e. those developing over greater than three months, are often asymptomatic, especially earlier in its course. Subacute effusions, which develop over several days to weeks, may present with a variety of symptoms, including dyspnea, generalized fatigue, peripheral edema, and chest pain. As the pericardial pressure surpasses the diastolic filling pressure, leading to cardiac tamponade, patients often experience lightheadedness, fatigue, anorexia, syncope, and may even progress to cardiac arrest (4,6). Acutely developing pericardial effusions can result in hemodynamic instability due to the rapid increase in pericardial pressure, leading to a faster onset of symptoms. Similar to late presentations of subacute tamponade, patients with acute cardiac tamponade typically present with lightheadedness, chest pain, syncope, rapidly declining mental status, and/or cardiac arrest (4,6,7).
Physical Exam
In addition to the clinical symptoms, specific physical examination findings can be critical for diagnosing cardiac tamponade. Common presentations include hypotension characterized by a narrow pulse pressure and reflexive tachycardia. A decrease in systolic blood pressure during inspiration of greater than 10 mmHg, referred to as pulsus paradoxus, may also be observed. Patient often exhibit marked jugular venous distention (JVD) and peripheral edema. Extremities may be cool and cyanotic, consistent with signs of cardiogenic shock. Heart sounds are frequently muffled or even absent, depending on the size of the effusion (4,6,7). Beck’s triad —consisting of hypotension, JVD, and muffled heart sounds—is particularly indicative of cardiac tamponade and should be assessed during the physical exam (8). Although not commonly used in emergency medicine, the central venous pressure waveform may show the absence of the Y-descent, a sign that can indicate tamponade physiology (9).
Diagnosis
The diagnosis of cardiac tamponade is often considered when a patient presents with the signs and symptoms of Beck’s triad. Unfortunately, this triad does not occur in the majority of patients, which limits its diagnostic utility (9). Imaging studies, such as computed tomography (CT) or cardiovascular magnetic resonance (CMR), can provide evidence of a pericardial effusion as well as associated chest abnormalities. An echocardiogram is a dynamic study that identifies pericardial effusions and specific signs suggesting cardiac tamponade (which will be discussed below); it is the recommended initial test in patients with suspected tamponade to evaluate the size, location, and degree of hemodynamic impact, with a Class IC Recommendation from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases (6). The diagnosis is further confirmed when there is improvement of hemodynamic abnormalities after pericardiocentesis, alongside clinical and imaging findings. Cardiac tamponade can also be confirmed with cardiac catheterization, though this is not commonly used in practice (6).
Management
Management of cardiac tamponade requires early identification of the diagnosis and removal of the pericardial fluid via pericardiocentesis. If tamponade is identified on point-of-care ultrasound (POCUS) and the patient is hemodynamically stable, the case should be discussed with interventional cardiology, if available, as the procedure is ideally performed in a catheterization lab where additional resources are available. If the patient is hemodynamically unstable or in cardiac arrest, emergent pericardiocentesis should be performed at the bedside. While traditionally a landmark-based procedure, ultrasound-guidance has several advantages; this technique will be discussed below in the POCUS section. Patients with acute cardiac tamponade due to traumatic cardiac injury or aortic dissection will require surgical intervention to address the underlying cause. In contrast, patients with subacute cardiac tamponade, particularly neoplastic effusions, are at higher risk for recurrence and may need a cardiac window procedure (6).
Administration of intravenous fluids in the acute setting, whether the patient is hemodynamically stable or unstable, has shown improved outcomes since this process is preload-dependent (10). Positive pressure ventilation should ideally be avoided in patients with cardiac tamponade, as it can decrease venous return to the heart and worsen the tamponade (7).
Point-Of-Care Ultrasound & Pericardial Effusions
For patients presenting to the emergency department with signs and symptoms concerning for cardiopulmonary pathology, POCUS is an invaluable diagnostic tool that enables clinicians to quickly gather critical information. When cardiac tamponade is part of the differential diagnosis, the initial step is to evaluate for the presence of a pericardial effusion, which is one of the primary clinical questions that cardiac POCUS aims to address. An effusion can be identified in any of the four standard cardiac windows, but it is most commonly visualized is in the parasternal long axis or subxiphoid views, as fluid typically accumulates in the most dependent portions of the pericardial sac. The phased array probe is generally used for identifying effusions in medical patients during cardiac examinations, while the curvilinear probe is commonly utilized in trauma settings during the FAST exam.
Figure 1: The pericardial Effusion (Left) separates the descending aorta from the pericardium, whereas the pleural effusion (Right) does not cross the descending aorta as the pleura attaches to the aorta at that point (13).
A pericardial effusion appears as an anechoic (black) stripe of fluid surrounding the heart’s borders, separating the hyperechoic (bright white) pericardium from the more neutrally-echogenic myocardium. The size of the effusions can be graded based on where the anechoic stripe is visible. Small effusions are usually only seen in the dependent posterior/inferior (far field) regions of the pericardium, while moderate and large effusions extend toward and into the anterior (near field) regions, eventually becoming circumferential.
A common pitfall when identifying effusions with POCUS is misinterpreting a pleural effusion as a pericardial effusion. The parasternal long-axis view is particularly useful for distinguishing between the two. A pericardial effusion appears as an anechoic stripe between the cardiac border and the descending aorta, as shown in Figure 1. In contrast, a pleural effusion tapers toward the descending aorta and does not cross over it, as the pleura attaches to the aorta, confining the fluid to the inferior/posterior region (11).
Another common pitfall is mistaking a pericardial effusion with an epicardial or pericardial fat pad. A fat pad may appear hypoechoic, similar to an effusion, or may contain echoes, but it moves with the myocardium and tends to be localized to the anterior aspect of the heart, unlike an effusion which usually involves the posterior, more dependent areas of the heart (11-12).
Sonographic Signs of Tamponade
-Chamber Collapse-
After identifying a pericardial effusion, the next step is to assess for sonographic evidence of cardiac tamponade. The earliest and most sensitive sign of tamponade is right atrial systolic collapse. Although this finding may be challenging to identify, it is best evaluated in an apical four-chamber or subxiphoid view, as these provide the most direct visualization of the right atrium. To accurately assess for atrial systolic collapse, observe the collapse of the right atrial free wall (or “scalloping”) when the tricuspid and mitral valves are closed, indicating ventricular systole (11). This is shown in Figure 2 below.
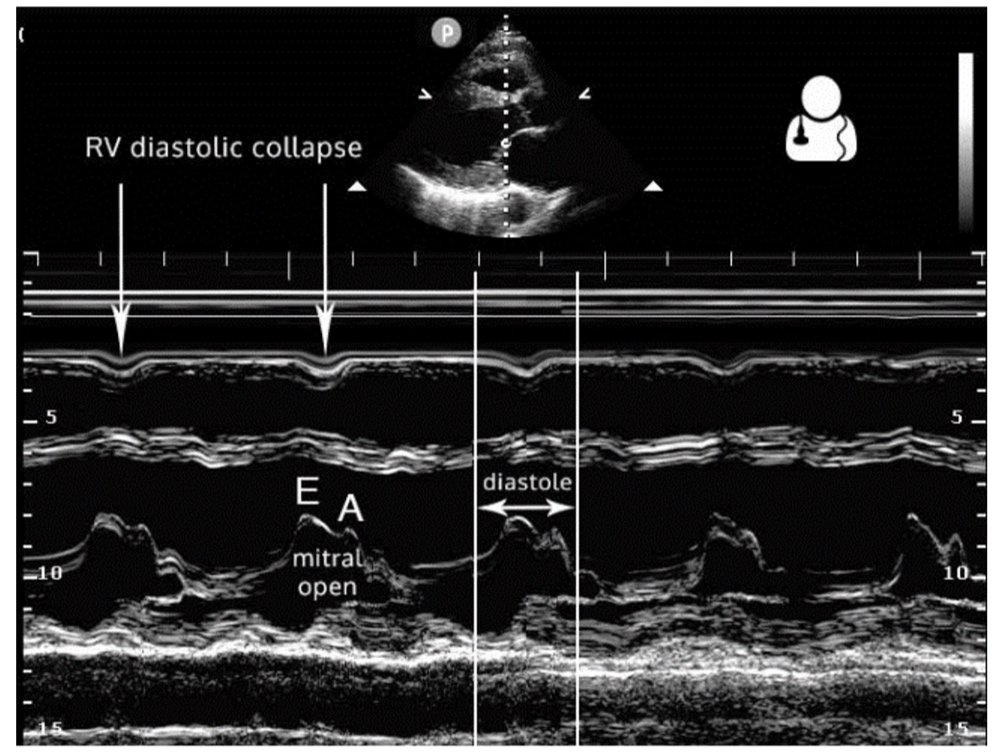
Right ventricular diastolic collapse is the classic and more specific, yet less sensitive, indicator of cardiac tamponade. The parasternal long-axis view is particularly useful for assessing this, as it provides a clear visualization of the right ventricle, aortic valve, and mitral valve. To optimally identify right ventricular diastolic collapse, it is important to capture an image that includes all three structures, as shown in Figure 3. Additionally, M-mode can assist in detecting cardiac tamponade by focusing on measurements through the right ventricular wall and the mitral valve. During ventricular diastole, the mitral valve opens between the E and A waves, corresponding to a dip in the right ventricular wall signal in the near field of the M-mode tracing, as illustrated in Figure 4 (12, 14).
Figure 2. Cardiac Tamponade with right atrial systolic collapse - the earliest, most sensitive sonographic finding (15).
Figure 3: Two images from the case demonstrating right ventricular diastolic collapse. Note the open mitral valve (Green Arrow), closed aortic valve (Blue Arrow), and collapsing right ventricle (Red Arrow).
Figure 4. Parasternal long axis M-Mode displaying right ventricular diastolic collapse (14).
A highly specific but less common finding, seen in only approximately 25% of patients, is left atrial systolic collapse (4). This may occur with extensive circumferential effusions, localized left-sided effusions or in patients with severe pulmonary hypertension where left-sided pressures are lower than those on the right (6, 11, 12). This can be seen in an apical, subxiphoid, or even parasternal view (demonstrated in this case - see Figure 5) where the collapsing left atrial free wall can be visualized with the closed mitral and tricuspid valves, thus confirming systole, can be seen. This is illustrated in Figures 5 and 6.
Figure 5. Left atrial systolic collapse, as seen in this case.
Figure 6: Apical 4 chamber view showing the systolic collapse of both the right and left atria (16)
-Plethoric Inferior Vena Cava (IVC)-
A useful adjunct to the cardiac views is assessment of IVC. A dilated or plethoric IVC (greater than 2cm in diameter, measured within centimeters as it approaches the right atrium) with little to no respiratory variation (less than 50% collapsibility with inspiration) indicates increased right-sided pressures, seen in a variety of conditions including tamponade. This is present in the majority of cases of tamponade but is not specific (17).
-Respirophasic Variation in Mitral and Tricuspid Inflow Velocities-
To further support the diagnosis of cardiac tamponade, measuring mitral and tricuspid inflow velocities can be beneficial. Flow through these valves varies with the respiratory cycle, and this variation is often exaggerated in the setting of tamponade; this is echocardiographic surrogate for pulsus paradoxus. Using pulse wave doppler in the apical four-chamber view, place the doppler gate just beyond the tips of the valve leaflets. The resulting waveform illustrates blood velocity over time, with variations in peak height reflecting changes in blood flow throughout the respiratory cycle. When examining the highest and lowest peaks, a variation of greater than 25% for the mitral valve and greater than 40% for the tricuspid valve inflow velocities supports the presence of tamponade physiology (18). This is commonly seen in tamponade but is not specific (17).
Figure 7. Mitral inflow velocity using pulse wave doppler (15)
Pericardiocentesis
Definitive management of cardiac tamponade is achieved through pericardiocentesis, which is ideally performed under ultrasound guidance. Several techniques can be employed, including subxiphoid, parasternal, and apical approaches (11). Regardless of the approach, the needle must be aligned in-plane with the probe marker to ensure direct visualization of the needle tip as it enters the pericardial sac, minimizing the risk of myocardial puncture, as illustrated in Figure 8. The various ultrasound-guided techniques, probe orientation relative to the needle, and potential complications are depicted in Figure 9. This will be discussed further in a future blog post.
Figure 8. In-plane dynamic needle guidance in a parasternal approach pericardiocentesis (19)
Figure 9. Approaches for ultrasound-guided pericardiocentesis (20).
Case Continued
The patient continued to remain hemodynamically stable throughout her time in the emergency department. The ultrasound findings above were discussed with the on-call interventional cardiologist, who agreed with the interpretation of sonographic tamponade. Given her stability, she was transported to the cardiac catheterization lab where she had an emergent pericardiocentesis performed leading to the drainage of 750 mL of maroon-colored fluid. The patient’s symptoms significantly improved after completion of the pericardiocentesis and she was admitted to the cardiology service for further management. The pericardial fluid was analyzed and was positive for malignant cells.
AUTHORED BY: RYAN STARKMAN, MD, PGY1
FACULTY EDITING BY: LAUREN MCCAFFERTY, MD
References
Al-Ogaili, A, Ayoub, A, Fugar, S. et al. Cardiac Tamponade Incidence, Demographics and In-Hospital Outcomes: Analysis of the National Inpatient Sample Database. JACC. 2018 Mar. 71(11):A1155.
Adamczyk M, Wasilewski J, Niedziela JT, Zembala MO, Gąsior M. Baseline characteristics, management and long-term outcomes of different etiologies of cardiac tamponade evaluated in a cohort of 340 patients. Kardiochir Torakochirurgia Pol. 2021 Dec;18(4):216-220.
Sharma NK, Waymack JR. Acute Cardiac Tamponade. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534806/.
Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003 Aug 14;349(7):684-90.
Reddy PS, Curtiss EI, O'Toole JD, Shaver JA. Cardiac tamponade: hemodynamic observations in man. Circulation. 1978 Aug;58(2):265-72.
Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015 Nov 7;36(42):2921-2964.
Reddy PS, Curtiss EI, Uretsky BF. Spectrum of hemodynamic changes in cardiac tamponade. Am J Cardiol. 1990 Dec 15;66(20):1487-91.
Sternbach G. Claude Beck: cardiac compression triads. J Emerg Med. 1988 Sep-Oct;6(5):417-9.
Ranganathan N, Sivaciyan V. Jugular Venous Pulse Descent Patterns: Recognition and Clinical Relevance. CJC Open. 2022 Nov 25;5(3):200-207.
Sagristà-Sauleda J, Angel J, Sambola A, Permanyer-Miralda G. Hemodynamic effects of volume expansion in patients with cardiac tamponade. Circulation. 2008 Mar 25;117(12):1545-9.
Noble VE, Nelson B. Manual of Emergency and Critical Care Ultrasound. Cambridge, UK: Cambridge University Press; 2014
Ma OJ, Mateer JR. Emergency Ultrasound, 2nd ed., McGraw-Hill Education, 2011.
Gibbons R. Pericardial vs pleural effusion. Temple Point-of-Care Ultrasound. February 8, 2018. Accessed July 2024. Available from: www.templepocus.com/tips-tricks/2018/2/3/pericardial-vs-pleural-effusion.
Smith B. UOTW #11 Answer - Core ultrasound. Core Ultrasound. July 28, 2014. Accessed July 2024. Available from: coreultrasound.com/uotw-11-answer/.
Royster K, McCafferty L. Intern ultrasound of the month: pericardial effusion and cardiac tamponade in COVID-19 pericarditis. University Hospitals Emergency Medicine Residency. Published January 31, 2022. Accessed July 2024. Available from: www.thelandofem.com/blog/2022/1/30/iusotm-tamponade.
Welch TD, Oh JK. Pericardial effusion, tamponade, and constrictive pericarditis. In: Nihoyannopoulos P, Kisslo J, eds. Echocardiography. Cham, Switzerland: Springer; 2018. Accessed July 2024.
Eke OF, Selame L, Gullikson J, et al. Timing of pericardiocentesis and clinical outcomes: Is earlier pericardiocentesis better? Am J Emerg Med. 2022;54:202-207.
Alerhand SA, Adrian RJ, Long B, Avila J. Pericardial tamponade: A comprehensive emergency medicine and echocardiography review. Am J Emerg Med. 2022; 58:159-174.
Echocardiography. TPA. Accessed July 2024. Available from: www.thepocusatlas.com/echocardiography-2.
Foamcast. Episode 54: The Pericardium. Accessed July 2024. Available from: foamcast.org/2016/08/08/episode-54-the-pericardium/.